The idea of ion exchange is not new. Scientists have been aware of the principle for a long time. However, it has only been since the start of the present century that the principle has been put to practical use. One area in which it has been highly effective has been in the treatment of water for the removal of hardness minerals and certain other contaminants. Ion exchange is the process through which ions in a solution are transformed into a solid which releases ions of a different type but of the same polarity. This means that the ions in solutions are replaced by different ions originally present in the solid. The physical separation process in which the ions are exchanged is not chemically altered.
Water that contains calcium and magnesium ions is also known as “hard water”. Hard water not only has an unappealing taste and odor, but it can also be unhealthy for you to drink. Hard water can cause scale build-up inside your water appliances and decrease the life expectancy of these appliances. One of the most commonly known ways to treat hard water is to use an ion-exchange water conditioner.
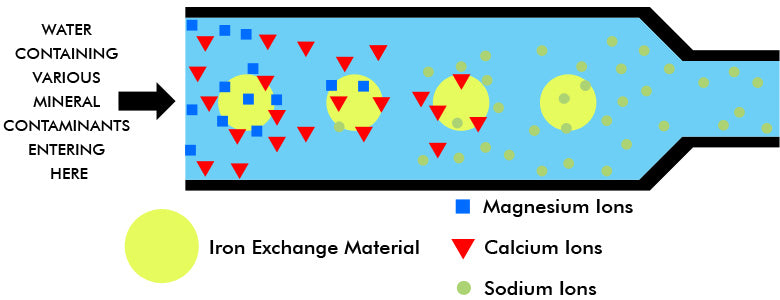
THE ION EXCHANGE COLUMN
HARD WATER ENTERING SOFTENER
All recognized household water softening equipment now on the market makes use of the ion exchange principle. Equipment using this principle contains a bed of permanent bead-like or granular softening material through which the water flows. As the water travels through the bed of ion exchange material, the hardness minerals are removed, leaving the water soft and more satisfactory for household use. The granules or particles of ion-exchange material in a softener are referred to as the bed.
The ion exchange material (usually resin beads or granules) consists of permanent insoluble anions, kept electrically neutral by replaceable sodium cations. Hard water contaminated with calcium and magnesium ions enters the exchange column or bed. As it flows through it, the magnesium and calcium cations in the water are drawn to the anions of the ion exchanger. The ion exchanger has a greater affinity for the calcium and magnesium ions than for the sodium ions. Therefore, the calcium and magnesium ions are absorbed, and a chemically equivalent number of sodium ions is released into the water. Thus, water containing the ions of calcium bicarbonate when it enters contains the ions of sodium bicarbonate as it leaves the ion exchanger bed. In brief, harmless sodium ions have replaced the trouble-producing hardness ions.
Ion exchange occurs literally billions of times between the material in the exchange column and the minerals in the water as softening proceeds. Water softening is one option for the homeowner to treat water problems. Calcium and magnesium decrease the effectiveness of water appliances by causing a film-like scale buildup. For example, have you ever noticed a white scale build-up on the insides of your water kettle? This layer is a buildup of calcium and magnesium, which will actually cause the kettle to use more energy to heat the water because of the thickened layer. This similar buildup occurs on the insides of your other home appliances, water pipes, washing machine, etc.
Water softening is an effective method to reduce calcium and magnesium from the water. When selecting a water softener it should be based on water analysis and assessment of the individual homeowner’s need. This is mainly determined by the levels of hardness in your water as well as the average amount of water consumption in your household. Contact your local city water source to receive more info on the quality of your water. If you are on a well, you may have to pay for your own individualized water report.



